[1] R. Frederick, Textbook of Military Medicine, Published by the Office of Surgeon General Department of Army, USA, p.pp. 1411997.
[2] J. Millerioux et al, "Evaluation of
In Vitro Tests to Assess the Efficacy of Formulations as Topical Skin Protectants against Organophosphorus Compounds," Toxicol. in Vitr., Vol. 23, pp. 127ŌĆō133, 2009.

[3] J. Millerioux et al, "
In Vitro Selection and Efficacy of Topical Skin Protectants against the Nerve Agent VX," Toxicol. in Vitr., Vol. 23, pp. 539ŌĆō545, 2009.

[4] C. Bignon et al, "Barrier Cream Based on CeO
2 Nanoparticles Grafted Polymer as an Active Compound against the Penetration of Organophosphates," Chemico-Biological Inter., Vol. 267, pp. 17ŌĆō24, 2017.

[5] Ernest H. Braue Jr., Development of an Active Topical Skin Protectant, USAMRICD-TR-16-03. pp. 1ŌĆō180, Feb. 2016.
[6] Sandra L. Fox et al, Emergency First Responders, Experience with Colorimetric Detection Methods,ŌĆØ Idaho National Laboratory U.S. Department of Homeland Security Under DOE Idaho Operations Office Contract DE-AC07-05ID14517. pp. 1ŌĆō33, 2007;10..
[7] S. W. Kim et al, "Study on the Formulations for Topical Skin Protectant against Liquid-Phase Chemical Warfare Agents," Journal of the Korea Institute of Military Science and Technology, Vol. 25, No. 2, pp. 210ŌĆō217, 2022.

[8] Carl T. Olson et al, "Evaluation of Compounds as Barriers to Dermal Penetration of Organophosphates using Acetylcholinesterase Inhibition," Toxicol. Lett., Vol. 55, pp. 325ŌĆō334, 1991.


[9] William G. Reifenra et al, "A Comparison of in Vitro Skin-Penetration Cells," J. Pharm. Sci., Vol. 83, No. 9, pp. 1229ŌĆō1233, Sep. 1994.

[10] OECD Guideline for the Testing of Chemicals. Skin Absorption: In Vitro Method, OECD/OCDE 428 Adopted :13 April. 2004.
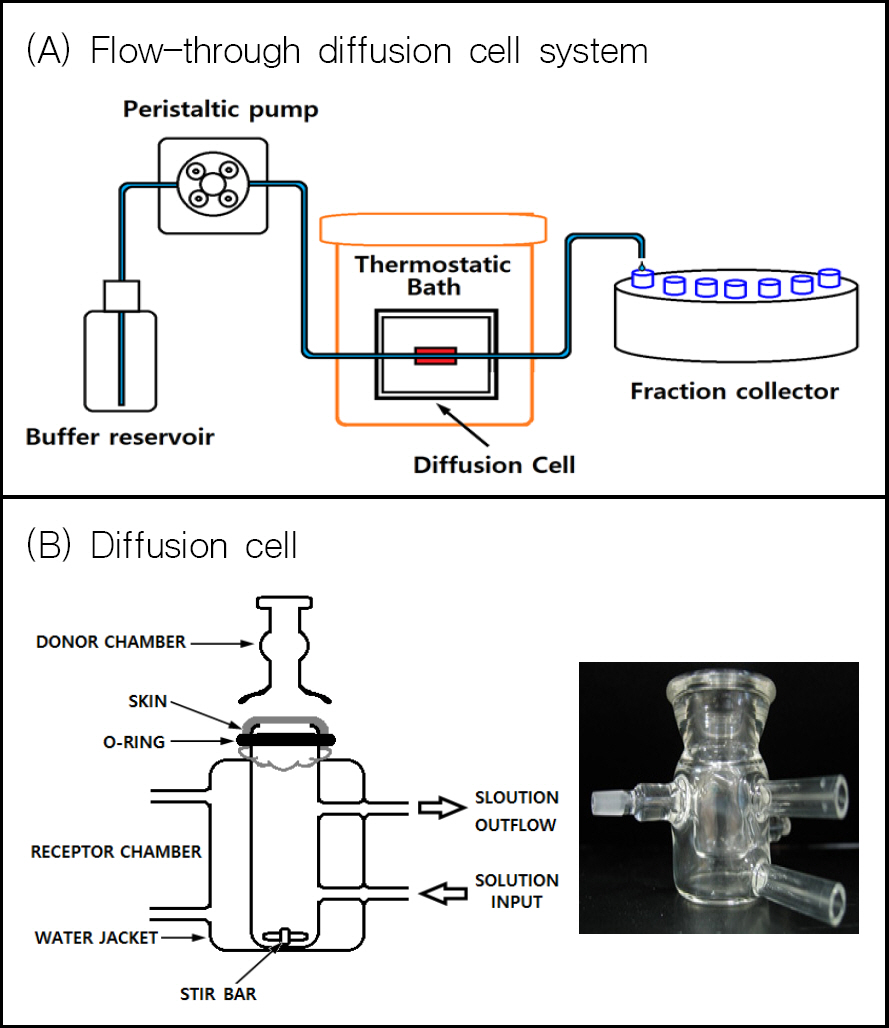
[11] S. Akhter et al, "Automated Diffusion Apparatus for Studying Skin Penetration," Int. J. Pharm., Vol. 21, pp. 17ŌĆō26, 1984.
[12] R. Bronaugh et al, "Methods for
In Vitro Percutaneous Absorption Studies," J. Pharm. Sci., Vol. 74, pp. 64ŌĆō67, 1985.

[13] W. Addicks et al, "Validation of a Flow-Through Diffusion Cell for use in Transdermal Research," Pharm. Res., Vol. 4, pp. 337ŌĆō341, 1987.

[14] J. Davies et al, "Further Development of an
In Vitro Model for Studying the Penetration of Chemicals Through Compromised Skin," Toxicol. in Vitr., Vol. 38, pp. 101ŌĆō107, 2017.

[15] Hoo-Kyun Choi et al, "Mathematical Analysis and Optimization of a Flow-Through Diffusion Cell System," Pharm. Res., Vol. 11, No. 4, pp. 595ŌĆō599, 1994.

[16] H. Dalton et al, "Absorption of the Nerve Agent VX (
O-ethyl-S-[2(di-isopropylamino)ethyl] methyl phosphonothioate) Through Pig, Human and Guinea Pig Skin
In Vitro," Toxicol. in Vitr., Vol. 20, pp. 1532ŌĆō1536, 2006.

[17] G. A. Simon et al, "The Pig as an Experimental Animal Model of Percutaneous Permeation in Man," Skin Pharmacol. Appl. Skin Physiol., Vol. 13, pp. 229ŌĆō234, 2000.

[18] C. R. Atchinson et al, "Development of a Guinea Pig Model for Low Dose, Long Term Exposure to Organophosphorous Nerve Agent," Toxicol. Mech. Meth., Vol. 14, pp. 183ŌĆō194, 2004.
[19] J. Wetherell et al, "Physostigmine and Hyoscine Improves Protection against the Lethal and Incapacitating Effects of Nerve Agent Poisoning in the Guinea Pig," Neurotoxicol., Vol. 23, pp. 341ŌĆō349, 2002.

[20] W. J. Addicks et al, "Validation of a Flow- Through Diffusion Cell for use in Transdermal Research," Pharm. Res., Vol. 4, pp. 337ŌĆō341, 1987.

[21] G. A. Simon et al, "Comparative Evaluation of Rivastigmine Permeation from a Transdermal System in the Franz Cell using Synthetic Membranes and Pig Ear Skin with
in Vivo-In Vitro Correlation," Inter. J. Pharm., Vol. 512, pp. 234ŌĆō241, 2016.

[22] FDA. 1997;Guidance for Industry: SUPAC-SS Nonsterile Semisolid Dosage Forms: Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls In Vitro Release Testing and In Vivo Bioequivalence Documentation.
[23] T. Lalain et al, Chemical Warfare Agents Decontamination Efficiency Testing Large-Scale Chamber mVHP Decontamination Evaluation, ECBC-TR-731, Edgewood Chemical and Biological Center. 2010.
[24] K. Lakshmi et al, "ZnO/PAN Nanofiber Catalyst for Photodegradation of Methyl Paraoxon and Its Toxicological Evaluation Utilizing Early Life Stages of Zebra Fish," Chem. Eng. J., Vol. 357, pp. 724ŌĆō736, 2019.
[25] L. SHANNON et al, "Review of Chemical Warfare Agent Simulants for the Study of Environmental Behavior," Critical Rev. in Environ. Sci. and Tech., Vol. 38, pp. 112ŌĆō136, 2008.
[26] L. Song et al, "Photothermal Graphene Fabrics for Ultra Fast Catalytic Degradation of Chemical Warfare Agent Simulants," J. Hazard. Mater., Vol. 393, pp. 122ŌĆō132, 2020.
[27] C. A. Moore et al, "Use of a Human Skin
In Vitro Model to Investigate the Influence of ŌĆśEvery- DayŌĆÖ Clothing and Skin Surface Decontamination on the Percutaneous Penetration of Organophosphates," Toxicol. Lett., Vol. 229, pp. 257ŌĆō264, 2014.


[28] C. A. Moore et al, "Percutaneous Absorption and Distribution of Organophosphates (Chlorpyrifos and Dichlorvos) Following Dermal Exposure and Decontamination Scenarios using
In Vitro Human Skin Model," Toxicol. Lett., Vol. 229, pp. 66ŌĆō72, 2014.


[29] S. Dachir et al, "Dermostyx (IB1)-High Efficacy and Safe Topical Skin Protectant against Percutaneous Toxic Agents," Chemico-Biological, Inter., Vol. 267, pp. 25ŌĆō32, 2017.

[30] T. Kadar et al, "A Topical Skin Protectant against Chemical Warfare Agents," Israel Med. Ass. J., Vol. 5, pp. 717ŌĆō719, Oct. 2003.
[31] N. Ophir et al, "Using the Skin Protective Lotion IB1 as a Substitute for Chemical Protective Gloves," Am. J. Emer. Med., Vol. 34, pp. 1986ŌĆō1990, 2016.